Pharmaceutical
Development Expertise
J2Fpharma team is making the most of its experience to support companies in their product development, focusing on Client objectives and timelines.
From project feasibility to dossier registration, our team is dedicated to support any request related to product development through a Quality By Design approach.
J2Fpharma delivers knowledge in formulation, manufacturing process, analytical development, validation and stability studies in line with international/local requirements as per targeted markets.
J2Fpharma provides end to end support for product development up to dossier registration via multidisciplinary project management. Our team can also support projects at specific stages of development, to solve any issue or answer to some strategic questions.
J2Fpharma expertise and services for pharmaceutical development are :
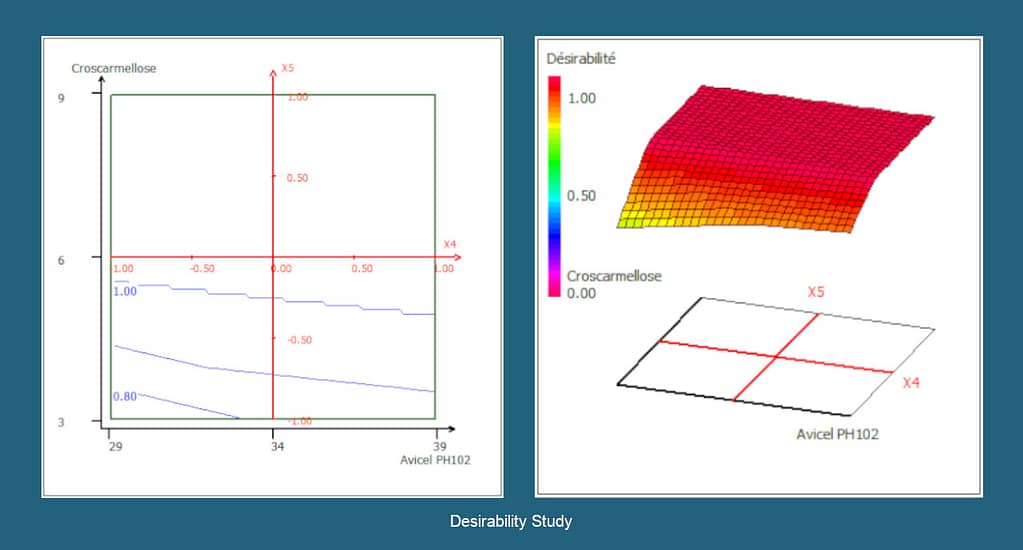
- Formulation using QbD approach and Design of Experiments (DoE),
- Analytical Development & stability studies,
- PK studies,
- Scale-up / Technology transfer,
- Quality audit,
- Chemistry.
For more information on project management click here :
Formulation,
QbD, DoE
Formulation design (all pharmaceutical forms) :
For NCEs, generics, lifecycle management (eg: fixed-drug combinations),
With a focus on Quality by Design (QbD),
Using tools such as Design of Experiment (DoE).
Analytics
& stability
Support for analytical development (using QbD) in line with regulation in force,
Analytical validation protocol & report review or writing,
Support for statistical treatment,
Stability studies (choice of packaging, results analysis, conclusions).
Scale up/
process
Process optimization (using QbD, DoE),
Support for scale-up / technology transfer,
Site transfer (participation to transfer, documentation writing or review),
Equipment installation, qualification (R&D and GMP).
PK
studies
Follow up of PK studies on behalf of the client (budget, partners, timelines, …),
Support on study design,
In vitro-in vivo correlation,
Identify & find partner CRO
(using our network and past experience).
Quality audits
& trainings
J2Fpharma can conduct quality audits of API sites, CRO, CMO partners (on behalf of the company) or in the frame of pre-inspection evaluation.
We also proposes trainings related to pharmaceutical development field
Chemistry
Active principle sourcing, synthesis review,
Do not hesitate to contact us for any questions
or to get more information about our services.
Exemple of conducted projects

Expert board for
a new formulation
“J2Fpharma was appointed to be member of a board with the aim of providing expertise for the development of a new formulation (NCE).”
read more
Analytical validation according to Brazilian guidelines
(RDC 166 & Guia n°10)
“J2Fpharma was in charge of the review of analytical validation data for 3 analytical methods (dissolution, assay, residual solvents) and…”
read more

Evaluation of impurities identification and degradation pathway
“J2Fpharma was appointed in order to expertise available data regarding degradation products observed during stability studies…”
read more
We make the most of our experience
to design suitable solutions and meet your needs.
35
Years experience in formulation of pharmaceutical products, analytical development & technology transfer
Strong knowledge on Qbd & DoE (team member of DoE congress)
Experience with
all pharmaceutical forms.
J2Fpharma provides necessary ressources, project management tools and reporting to meet your targets & timelines


